Expand Your Diagnostic Portfolio - Rapid Tumor Marker Testing with Poclight CLIA Solutions
The Rising Global Cancer Burden
According to the latest report from the World Health Organization (WHO) and its cancer agency, IARC, there were approximately 20 million new cancer cases and 9.7 million deaths worldwide in 2022, with around 53.5 million people alive within five years of a cancer diagnosis.
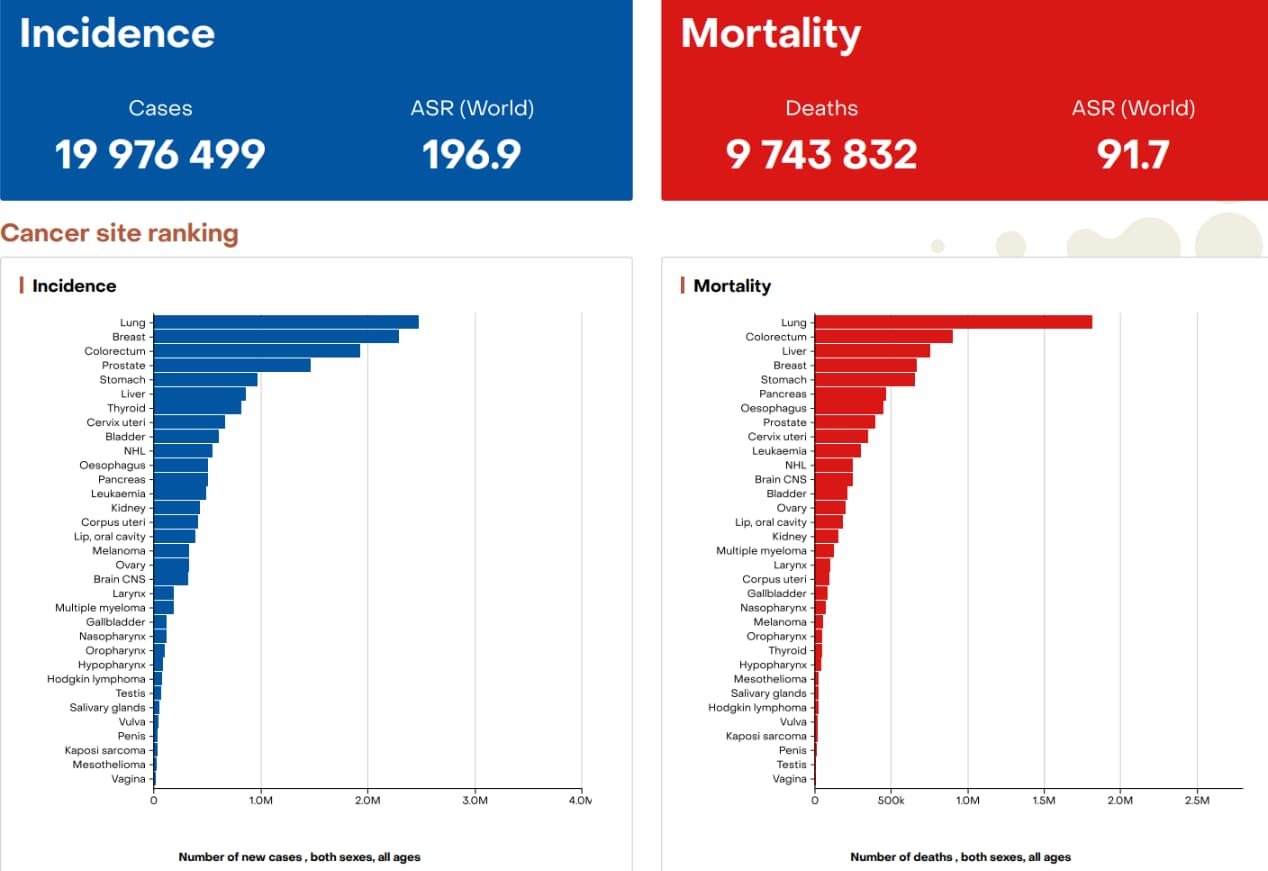
Lung, breast, and colorectal cancers were the three most common types, with lung cancer remaining the leading cause of death. Low-income countries face higher mortality due to late diagnosis and inadequate care.
By 2050, global new cancer cases are projected to reach 35 million, with the largest increases in low- and middle-HDI countries. WHO calls for strengthened cancer care coverage and investment to ensure accessible, high-quality cancer diagnosis and treatment for all.
The Importance of Early Screening
● Up to 40% of cancer-related deaths could be prevented through early detection and timely intervention. (The Lancet Global Health)
● Developed countries show higher screening coverage, while accessibility remains a challenge in developing regions.
● The trend toward multi-cancer early detection (MCED) is accelerating globally, promoting a more comprehensive approach.
For Breast Cancer Awareness Month (October)
● Breast cancer is the most common cancer among women worldwide.
● Self-examination is recommended starting at age 20.
● Women over 40 are advised to undergo imaging combined with biomarker screening every 1–2 years.
● Early detection (Stage I) can lead to a 5-year survival rate exceeding 90%.
Recommendations and statistics are based on sources, including WHO guidelines, the American Cancer Society (ACS), EUSOMA guidelines, and global cancer data from GLOBOCAN/IARC and the NCI SEER database.
2. Key Tumor Markers and Their Clinical Significance
|
Biomarker |
Associated Cancer Type(s) |
Clinical Use |
|
PSA |
Prostate Cancer |
Routine screening marker; early detection and therapy monitoring |
|
CEA |
Multiple Cancers (colorectal, lung, breast, etc.) |
Broad-spectrum marker for diagnosis aid and recurrence monitoring |
|
AFP |
Liver Cancer |
Key indicator for hepatocellular carcinoma screening and differentiation |
|
CYFRA21-1 |
Lung Cancer (especially squamous type) |
Supports diagnosis and treatment evaluation |
|
CA19-9 |
Pancreatic & Gastrointestinal Cancers |
Digestive system malignancies and therapy monitoring |
|
CA125 |
Ovarian Cancer |
Screening and disease progression evaluation in female patients |
Combining markers increases sensitivity and specificity by compensating for individual testing limitations:
● Ovarian cancer: CA125 + HE4 achieves 93.8% accuracy.
● Pancreatic cancer: CA19-9 + CEA + CA125 raises sensitivity to 82%.
● Prostate cancer: f/t PSA ratio reduces false positives by 30% in the gray zone.
3. Poclight’s Innovative Diagnostic Solutions
In today’s evolving healthcare landscape, laboratories and diagnostic providers are seeking faster, more reliable, and accessible cancer testing solutions, yet traditional immunoassay systems often require high costs, long turnaround times, and complex operation.
Poclight offers a comprehensive portfolio that empowers both distributors and laboratories to expand access to high-quality cancer diagnostics anywhere, anytime.
1) Advanced Platform: C5000 Dry Chemiluminescence Immunoassay Analyzer (Dry CLIA Analyzer)

2) Featured Tumor Marker Panel
✓ Combined tumor marker testing: PSA, CEA, AFP, CYFRA21-1, CA19-9, CA125
✓ Covers multiple major cancer types (liver, lung, prostate, gastrointestinal, ovarian)
✓ Fast, accurate, and suitable for hospitals, health centers, and primary care facilities
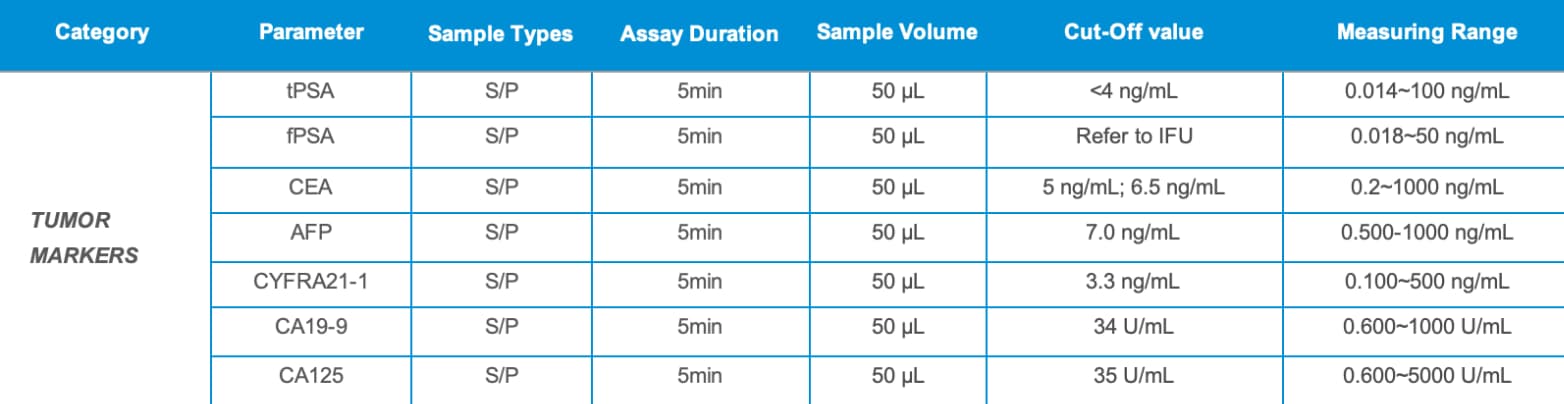
✓ Rapid detection in just 5 minutes, minimal sample volume of 50 µL, simple operation, low cost, high-quality CLIA methodology, innovative CRET patented technology, precise results, and a wide linear detection range.
Ease of Use: Minimal training required
More tumor marker and other assays in progress
Poclight Biotech is actively seeking global partners who share our vision for accessible, high-quality diagnostics.
Reach out to us to explore collaboration opportunities or to learn how our solution can fit into your portfolio.