Poclight CLIA POCT in Preventive and Diagnostic Health Checkups
1. Introduction
Annual preventive screenings are more than just a routine. They help us detect subtle changes in our bodies before they become serious.
Catching potential issues early allows us to take action sooner, manage risks effectively, and maintain our overall well-being.
Wondering which tests are most important for a comprehensive view of your health? Today’s article may provide some guidance on essential screenings to consider during your next routine check-up.
2. Routine Preventive Screening for General Population
Preventive screening covers multiple body systems, from metabolic and cardiovascular health to thyroid function and nutritional status. Below we categorize the most relevant tests for general adults, explaining their purpose, recommended population, and associated health risks.
|
Category |
Markers |
Purpose |
Recommended Population |
Related Conditions / Risks |
|
Allergy / Immune Sensitivity |
Detects allergic sensitization |
Allergy-prone individuals Family history of allergy |
Allergic rhinitis Asthma Other allergic conditions |
|
|
Cardiovascular |
BNP NT-proBNP hs-cTnT |
Evaluates cardiac stress and early myocardial risk |
Adults over 40, adults with family history of heart disease |
Early heart strain, myocardial injury |
|
Thyroid Function |
TSH FT3 FT4 |
Evaluates thyroid hormone balance |
Individuals with fatigue, weight changes, or metabolic concerns |
Hypothyroidism Hyperthyroidism |
|
Glucose |
HbA1c Insulin |
Monitors long-term glucose control |
Adults at risk for metabolic disorders, overweight, or with lifestyle-related risk factors |
Diabetes, Prediabetes |
|
Nutritional Status |
Ferritin 25-OH VD Active B12 |
Assesses iron and vitamin status to detect deficiencies |
General adult population, elderly, individuals with restrictive diet |
Anemia, vitamin D deficiency, vitamin B12 deficiency |
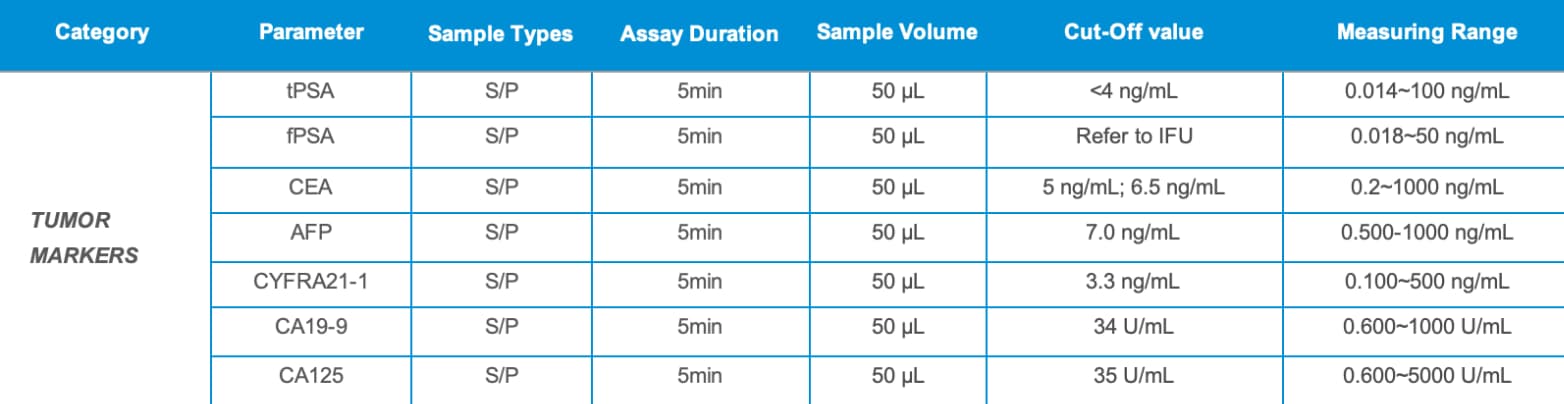
Risk-Based Tumor Marker Testing
Cancer screening using specific biomarkers is not part of routine check-ups for the general population. Instead, these tests are recommended for individuals at higher risk or under physician guidance to support early detection and targeted health monitoring.
|
Category |
Markers |
Purpose |
Recommended Population |
Related Conditions / Risks |
|
Cancer Screening |
tPSA fPSA CEA AFP CYFRA21-1 CA19-9 CA125 |
Supports risk awareness and baseline health monitoring |
Individuals with relevant family histories or personal cancer histories |
Prostate cancer, liver cancer, lung & digestive system cancers, ovarian cancer |
3. How Poclight CLIA POCT Supports Preventive Screening Programs
With the advancement of CLIA Point-of-Care Testing, high-quality laboratory diagnostics can now be delivered rapidly at the point of care, making multi-parameter screening feasible in routine health examinations.

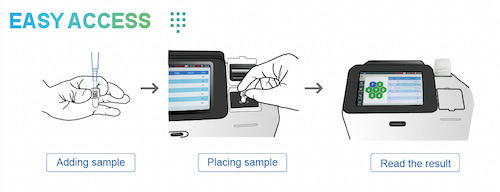
Poclight C5000 micro CLIA analyzer helps laboratories and clinics deliver laboratory-grade results with point-of-care efficiency, offering:
● Rapid results: delivers accurate results in 3-15 minutes across 40+ tests
● Stable reagents: freeze-dried reagents storable at 2-30°C for up to 18 months
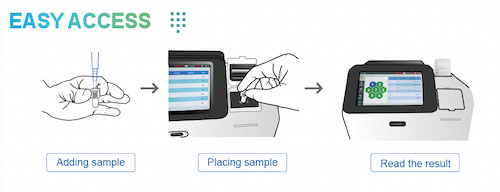
● User-friendly operation: simple 3-step workflow within a compact 8.5 kg analyzer
For partnership and distribution inquiries, please contact us.